METHODS:
- Deliver 2g/hr, 24 hr/day, 7 days/week, for 1 week, for at least 2 normal (non-cancerous, moderately healthy) adults of average weight and size, using convention sodium ascorbate IVC solution.
- Repeat for the 2nd week using proprietary buffer-stacked™ ascorbate solution again at 2g/hr. This incorporates magnesium ascorbate and potassium ascorbate along with the sodium ascorbate. The advantage to the user with this fluid is that it takes up 1/2 of the space.
- Measure effect on:
- blood composition (ascorbates, electrolytes, CRP, pH, platelets, etc)
- blood pressure
- urinalysis
- quality of life (QLQ-c30 test)
- 8-point functional bio-marker test
- Compensate for electrolyte losses if they be any.
- Measure scurvy rebound following going “cold-turkey” after an entire week of the protocol.
- If normal persons experience no negative side effects, prescribe the same treatment for 1 person with cancer meeting the criteria specified below in section: Patient Exclusion Factors.
- Perform bacterial tests on blood samples from subjects after 3-7 days of treatment, compared to blood from 2 other kinds of subjects: those consuming 1g/ hour up to bowel tolerance, and those not supplementing. All samples after being drawn will be intentionally infected with the same streptococcus bacteria and allowed to incubate for 1 hour under ideal conditions. Additional doses of the bacteria will be added hourly until the infection is detected in any of the samples. Additional measurements will be periodically conducted after that point.
OBJECTIVES / GOALS:
- Determine effect that sustained IVC has on any of the ascertained parameters and determine protocol modifications as needed to maintain acceptable levels for these tests.
- Optimize flow-rate adjustment criteria, as this is operating in an unusual high-osmolality / very-low rate situation
- Identify increased immunity, if any, compared to control for the streptococcus bacteria infected blood samples, assessing the impact this protocol has for the on-label use of vitamin C as an immunity booster.
- Prove stabilization design for full week use of IV catheter.
SECONDARY OBJECTIVES
Presuming that the objectives were satisfactorily met with the initial 2 non-cancerous patients, resulting in a final protocol that did not diminish their QLQ-c30 score or any of their functional responses within 1 standard deviation, the same protocol will be tried on a cancer patient.
However if further assessment is required to provide and validate a final protocol that will not diminish the QLQ-c30 score or functional responses within 1 standard deviation, the protocol will be further adjusted and validated on a non-cancer patient.
- Collect the same information as for the 2 previous patients.
- Measure the cancer response in terms of critical cancer markers.
Study Design Information
- IVC Book Team (IVCbook.com)
- Experimental
- Establish Safety Limits | Part of an ongoing study (provide parent study below) | Preliminary investigation | Identify therapeutic thresholds
- no
- yes
- not blind
-
● Scurvy as it relates to cancer (wherein scurvy is endemic)
● Conditions in which improved immunity is beneficial
● Conditions for which accelerated collagen synthesis or improved human functioning is beneficial - 24/7 IVC Family of Studies
-
With respect to the time-dependent cytotoxicity to cancer (NAD depletion):
(1) Original long-duration protocol (50 patients):
http://www4.dr-rath-foundation.org/NHC/studien_pdf/old/the_orthomolecular_treatment_of_cancer.pdf
(2) Addition information on the original 50 patients plus 50 patients:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC431183/pdf/pnas00040-0366.pdf
(3) More information with a revised set of 100 patients (mostly the same patients):
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC336151/Recent studies demonstrating cancer-cell energy depletion at mobile pump ascorbate doses:
(1) “vitamin C inhibited energy metabolism through NAD depletion, thereby inducing cancer cell death.” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4563566/
(2) ATP levels demonstrated a dose-dependent decline with ascorbate treatment. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2807999/
(3) “pharmacologic ascorbate concentrations diffuses into cells and mediates toxicity in sensitive cells by ATP depletion”. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1885574/
(4) “severely target energy production”: Vitamin C shuts down the glycolic pathway for cancer cell energy production. Combined with Doxycycline, which shuts down the OXPHOS pathway it demonstrated cancer cell energy depletion. 10.18632/oncotarget.18428
(5) Vitamin C causes NAD+ depletion (energy depletion), thereby inhibiting Cancer Stem Cell (CSC) activity exhibited by NADH expression, 10 times better than conventional methods. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5400535
(6) “ascorbate-induced oxidation … to decrease the ATP level in the cell”. https://www.ncbi.nlm.nih.gov/pubmed/12914777
(7) “inhibits GAPDH by both post-translational modifications and NAD+ depletion ultimately leading to an energetic crisis and cell death”. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4778961/
(8) “ascorbic acid concentrations produce extracellular H2O2, which can diffuse into cells, deplete ATP in sensitive cells, and thereby cause cell death”. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1885574/
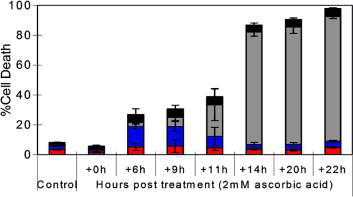
(9) See chart above, 2mM ascorbate dose takes at least 14 – 22 hours to fully starve cancer to death of energy (Note that it will be considerably longer in the human body due to hypoxic conditions). https://www.ncbi.nlm.nih.gov/pubmed/16157892
(10) “Practitioners should pay more attention to the cumulative vitamin C effect instead of the vitamin C concentrations”. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4031610/ - Other Cytotoxic Vitamin C protocols: (1) Conventional IVC (up to 30g/hr, for 2 hrs); (2) Liposomal C. See chart in description.